I was triggered into this post by this news article titled extreme TB in The Hindu which claimed that
“Nearly 1.3 lakh DR-TB patients need treatment but the Health Ministry has only 10,000 doses of Bedaquiline and 400 doses of Delaminid, obtained as ‘donations’ from Janssen (US) and Otsuka Pharmaceuticals (Japan), the respective manufacturers.”
I apologize for this troll-bait of a headline which wouldn’t please journalist, but it should lead them to look at Drug resistant TB from another perspective.
I pointed out factual inaccuracies in the post and rebutted that only 3- 10% of the Drug resistant patients TB patients have extreme Drug resistant TB condition in which these drugs are recommended by WHO. Further, Bedaquiline have had higher deaths in patients in which it was given though it cured more TB patients in one clinical trial though it has shown promise in other non-randomized and non-controlled studies since then, while the other drug Delaminid simply was ineffective in other trial and WHO updated its position on this via an urgent notification. I found this disease mongering(giving 10 times estimate) unacceptable and even worse , that there was no mention of side-effects of tone drug and inefficacy of other.
So instead of correcting their facts, as usual the tweleb journalist-activists who have reporting on TB for ten years now started lecturing me on ethics and citing wrong data basically comparing total number of cases to total number of deaths.

But these run-ins are common on twitter and i thought this was just advocacy journalism which is quiet common now a days. However things became trickier when other article by a Assistant Prof at NALSAR Hyderabad, prashant reddy who works in field of patents suggested that the compassionate use was a de-facto clinical trial where no liabilities for deaths would be borne by companies.
Considering people have gone to courts for using these drugs, the extreme disease mongering could be harmful. I thought I should blog about how the activism-journalism and unmonitored clinical trials in a drug with adverse intial phase 2 data in terms of mortality could in fact lead to a night mare scenario for the same MDR TB cases.
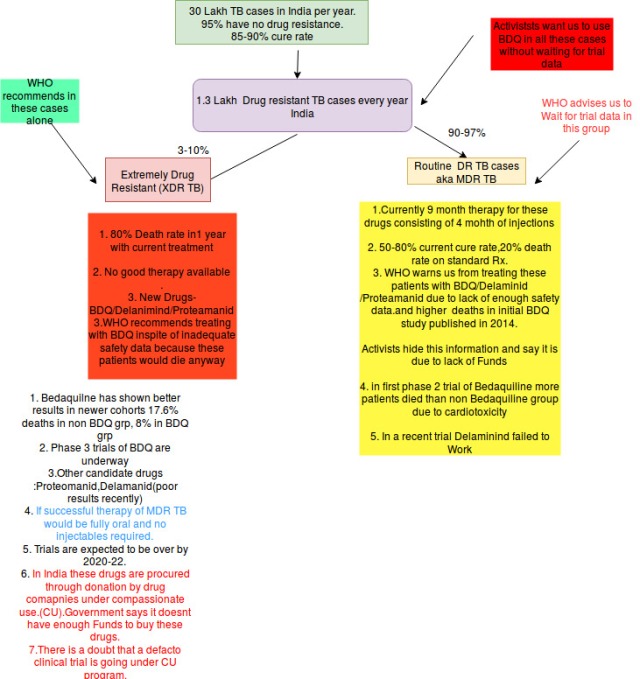
Short answer : Read this summary slide:
Long Answer:( Around 7-10 minutes read)
Now I will provide a perspective about TB in layman’s language in an FAQ format so that it will help you make up your mind as well as give an idea about drug development process and policies and conflict of interests in medicine,,
Q.. What is TB ? How many people suffer from it?What is Drug resistant TB?
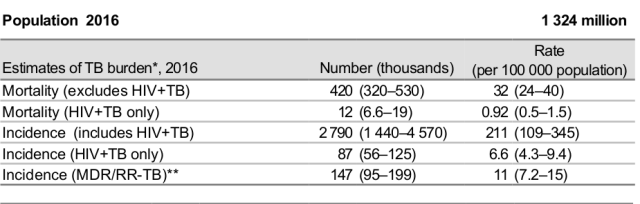
A. TB is chronic infective disease caused by a bacteria Mycobacterium tuberculosis which most commonly starts in lungs but has insidious symptoms but can happen in other parts of body. Luckily with established oral drug combinations(Four drug Combination) it can be treated in six months in most of the cases.In India almost 30 lakh patient suffer from TB every year and out of them 4.2 lakhs die. (17% Case Fatality).
Most of the cases of TB have 70-90% cure rate however However 84000-147000 patients out of these 30 lakh case(3-10%) have drug resistant TB (resistance to at least 2 out of 4 conventionally used drugs ) in which mortality rate is around 20-30% in 3-4 years and there is only 50-70% cure rate .
Diagnosis is established mostly by staining of sputum samples of patient, the culture of sputum and new rapid diagnostic PCR modalities like CB-NAAT.
While sputum slide tests can diagnose lung TB ,they cant diagnose resistance, for which we need either sputum AFB culture(done at special labs, takes 4-6 weeks) or CBNAAT(provided free at many government centres, would cost around 1500 rupees in market).

Q. I have heard Multi drug resistant TB is on rise? Why should we worry about it?
It is not on rise but was underestimated and improvement in rapidly diagnostic modalities like gene expert ( which can diagnose lung Tb in 2 hours and detect resistance as well) have improved its estimates. As i said from 3%(newly diagnosed) to 10% cases(previous treated cases who didn’t respond/left treatment ) would have drug resistance.
Now as opposed to conventional TB which can be treated with four drugs, it needs to be treated with 9 months of therapy and six drugs . Three new drug classes are required here- one of them is an injectable drug ( Kanamycin here which belongs to aminoglycoside group of drugs) ,other a Fluoroquinolone(Moxifloxacin here) and one a thioamide(Prothionamide) which can have range of side effects from hearing loss to Kidney injury. The most troublesome aspect of this apart from side effects is the fact that you require injections for almost four months and frequent hospitalisations.
If you think this in quite inconvenient, consider that prior to 2016 the guidelines were to treat for 18-24 months with almost six months of injections.
So Activists do have a point about inconvenience and side effects.
As I said this regimen has cure rate of around 50-80% and mortality from 20-30% depending on patients.
And some TB bacteria have become resistant to even these injectable and oral drugs(fluoroquinolone) this types of TB is called extremely drug resistant TB(XDR TB)
In This type of TB almost 80% of patients would die without conventional therapy and there is almost one third cure rate..

Q. I am scared now, one of injections and other of high death rate in particular in so called XDR TB? Arent there new medicines on horizon?
A. Before I talk about specific drugs -let me briefly walk you through process of drug development.
A drug goes through briefly four stages before it is brought out in market by drug companies:

Now it may seem easy, but process for drug development is long , arduous and a drug may fail at any stage.(meaning fail to show benefit , have harms etc). Typically this takes around 5 to 10 years. Imagine this scientists dedicate entire careers for developing a drug and it might fail. Almost 90% of promising molecules/drugs which show promise in labs in phase 0 fail to make it to phase 3 or phase 4. To hedge their failures, drug companies have multiple molecules in pipeline.
Now back to TB-
In TB, for 15 long years the process of drug development was stuck at phase 0 and 1, despite the fact that drug resistance was on rise( around 3% to 10%). It was an extremely frustrating time. Resistant TB cases were typically treated with combination of molecules(many of them injectables and depending upon resistance profile) for long period of 1.5-2 years
Then around 2010-11, many new promising molecules made it past phase 0 and phase 1
Q.Tell me More
OK.
Good news first :
Sure, New Molecules have come up and the good news is that they are oral. They are protagonists of our story as well-
Bedaquiline(B) ,pretomanid(P) and Delaminid(L) these have come up in last few years(after almost 15 years of no results in TB molecule development) and they are promising candidates.
Now not only are they oral but they showed showed promise even in TB resistant cases
Imagine a world free of injectables , Multi Drug Resistant TB and XDR TB could be treated with only three months of oral drugs.
Do you think I am selling you a pipe dream? Not at all .This is What happened in Hepatitis C recently!
One year of therapy with injectable interferons was completely replace with oral drugs which are given for almost 2-3 months only. A veritable miracle!
Bad News now:
Life is tough
Bedaquiline which killed most TB bacteria in petridishes , again killed the drug ressistant bacteria in 80% of patients compared to 57% in oral group but it ended up killing more people in first large single centre phase 2 study in 2014 .
while in other study in 2017 Delaminid performed worse than non Delaminid group(due to old bug a boo-Drug resistance) had no benefit , and in fact WHO issued urgent notification regarding this.
But since these were promising molecules , and number of deaths in Bedaquiline group were small though higher, it was thought that it might be a random anomaly so FDA gave a conditional approval that you can test them in more patients in phase 3 trials and see their results before we roll out for population.
However as i told there are some problems – before approval these drugs require a large multi country phase 3 trial and these are expected to be over only by 2022..
Q. What does “kill more people while curing more people” mean in Bedaquiline ?
Well, a drug is measured through 2 -3 outcomes in a clinical trial.
First is primary outcome namely death or hospitalisation which is what concerns us.
Second is specific outcome or surrogate end point which a doctor desires in his hospital – example in bedaquiline’s case clearing up TB bacteria from resistant patient’s sputum.
Generally Drugs perform on both First and second criteria but typically benefit on second criteria is more than first as obviously it is easier to show benefit on specific criteria rather than a general criteria like death.
Occasionally drugs show benefit on specific criteria while they show no benefit or even harm on primary outcome. due to unknown side effects which become evident during human studies only.
Read more here about one such study here
But number of deaths in placebo group in Bedaquiline study was small and hence it was decided that these events might be random.

So FDA(regulatory body of trials) advised to carry out larger multiple country phase 3 studies before approving Bedaquiline for MDR TB.
Q. I agree about doubts but in extremely resistant XDR TB mortality is high upto 80%, as opposed to simple MDR TB where it is 20% as you told with conventional therapy. shouldnt these patients be given these drugs ?
A. Yes, actually this is what FDA and WHO did they gave accelerated approval for Bedaquiline . Bedaquiline is now a first line in XDR TB cases(where they will die anyway without therapy) while allowing for larger phase 3 trials in Drug resistant cases which would be finished by 2020-22 subject to condition that drug companies will report about side effects yearly in respective countries.
WHO updates in position yearly based on data from other countries- cohorts and RCTS. Here is latest statement from WHO on Bedaquiline from march 2017

We can see clearly here that WHO doesn’t recommend Bedaquiline for MDR TB cases but for XDR cases- which are about median of 3-10% cases of MDR TB or patients where these MDR TB drugs cant be given due to many side effects.
To put things in perspective while 84000-1.47 lakhs of patients have drug resistant TB (MDR TB) almost 10% that is around 10-15 k might be having XDR TB. These patient do need Bedaquiline.
Update : Indian RNTCP Program and WHO now recommends using it in pre-XDR cases cases as well i.e. (MDR TB+ either of FQ/injectable drug ) which lead to additional 10% cases. ( Around 20% cases in India TB esistancee survey have XDR+Pre-XDR ).Thus best estimateof number of cases needing BDQ will be around 25-30000
Q. So how is Indian government managing this?
These drugs as i told you are extremely costly upto 30000 dollars/year.
Indian government says it doesn’t have money for drugs which is a shame and Activists have a point here.
Almost 5000-15000 patients of XDR TB are vulnerable without these modern drugs.
But the figure is not 1.5 lakhs as is informed by advocacy journalists and Activists.
Currently these drug companies donate the drugs to India about 10000 patients on bedaquiline and 200 on Delanimid under compassionate use.
Eventually these companies want the approval for not only extreme (1% of Total TB cases) but entire MDR TB cases(10% cases) .
But while in other countries apparently while these companies are doing clinical trials , In India it appears that drug trial data is being given and government is bearing liability for harms in name of compassionate use
Appears Nothing is free in world.
Further, particularly in India where almost 1000 -2000 people die from malaria due to lack of relatively cheap artesunate (1000 rupees) of malaria which causes mortality reduction by 30% or even due to diarrhea due to lack of ORS or IV Fluids or even due to lack of oxygen.
though it is not a binary, I think there should be a graded response to health crises according to cost effectiveness and efficacy.
Q. In recent trials Bedaquiline has shown benefits with mortality only in range of 10% compared to 20% in non Bedaquiline group
A These are not trials, These are observational studies(MSF,EDRWeb )while they are helpful,history of clinical trials tells us not to trust observational studies or small single center trial as definite evidence..
On basis of observational studies and advocacy groups millions of post menopausal women were given hormonal replacement therapy in nineties. End results surprised us all- in the WHI trial There was 20-30% rise in occurrence of heart attacks and stroke with 30% rise in breast cancer.
God knows how many innocent females were killed by this unblinded therapy
Q. Similar strategy was used to suppress access to HIV Drugs in US in nineties . you are playing the same game?
A. No, I am not. Even the win of activism in HIV is a grey story and has similar history but not in the way you want to portray.
Initial HIV drugs had same problems as MDR TB drugs now , toxicity,inconsistent efficacy,expensive.
So US government restricted access only to the patients with full blown AIDS( CD4 count <200) with significant mortality awaiting substantial data like it has done for Bedaquiline for XDR TB (not for MDR TB). . If the same toxic drugs would have been used in milder forms of HIV it would have harmed more than helping.
Now with time newer set of molecules have come , toxicity is minimal and prices are cheaper ..so what do we do now we prescribe HIV medicines to all HIV patients regardless of CD4 count. So it is a victory of iteration,safety and science not of activists alone as they would try to project(they however helped tremendously in reducing stigma of HIV).
My position on role of Bedaquiline is same if it establishes itself in phase 3 trial with success and minimum side effects and if indeed the initial results were an anomaly , i would be more than happy.
You can read more about history of evidence based medicine and HIV activism here.
Q. Professor Furin who teaches at Harvard says Indian government and Public Officials should be ashamed of saying that they are trying to save money by restricting access to MDR TB drugs. Dr. Udwadia also says so
A.Professor Furin rightly argued for access to BDQ in XDR TB case
Professor Furin cleverly under the barrage of rhetoric hides the fact about safety concerns with Bedaquiline, Jha the public health official never said anything about resistance at least on twitter, he just reiterated WHO guidelines
If Professor furin ,Dr. Udwadia or the journalists concerned have issues they should talk to WHO or re-read history of clinical trials, we dont want disasters like hormone replacement to happen again.
I am trained to do Evidence based medicine, I would look at Cochrane database,WHO guidelines and RCTs to practice my medicine rather than hear what a prominent doctor says no matter how well reputed they are.
Q. There have been anecdotes and observational studies that Bedaquiline’s side effect profile is over-estimated
A. Great, but I wont trust them.
At a minimum I would rely upon an independent audit of phase 3 trial by FDA .
The reason for this is :
- The sample size calculations of The clinical trials are not powered enough to detect side effects( which are rarer) but for effect detection
- The Drug companies have a history of hiding side effects once they are out in market in phase 4 as seen in case of Merck which sat over side effects of vioxx ,killed almost 30,000 people so that they could recover their money and arm-twisted a leading US cardiologist Eric Topol for criticizing them
Now Consider this , Bedaquiline side effects were apparent even in smaller subgroup rather than vioxx whose side effects were evident only in phase 4, so it is likely that danger is higher and should be subject to scrutiny at least at level of phase 3 trial even though it is used in sicker MDR TB patients.
Q. But Drug resistant TB is on rise and they will spread and kill us all before these drugs are widely available..
A. Drug Resistant TB was underestimated before but thanks in large part to rapid diagnostic methods like CBNAAT it is diagnosed within 2 hours, Government of India to its credit has rapidly scaled up rapid diagnostic technique . I submitted my thesis in CBNAAT (diagnosis of drug resistant TB in HIV patient) in 2013 and have presented research and papers on it , at the time there were only 14 centres to diagnose drug resistant TB rapidly in India( as opposed to culture which takes 4-6 weeks and has been available since long) now there are 628. While due to this rapid expansion number of DR Tb patients diagnosed has gone up from 17000 to 33000.
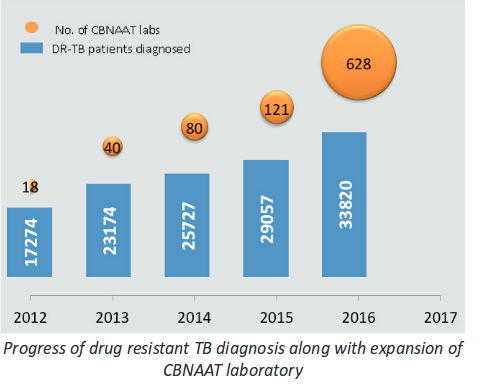
In that sense the TB program is victim of its own success, while it doesnt have a program for purchasing drugs for expensive XDR drugs and relies on donation(not for MDR TB routine cases -where there is a program for many years) .
In My opinion if the government were not serious about Drug resistance TB it would have not expanded the CBNAAT program as well.
Q. But major papers like The Hindu and the scroll have mentioned that nothing is being done for 1.3 lakh drug resistant TB patients. I see from your tweets that you have right of center views,Is it your plan to disparage them?
A. No, first it is an ad-hominem attack so i wont address that. But my view is that on this particular issue Journalists are engaging on advocacy journalism that is practising journalism with a non-objective view point, Now it happens all the time in political journalism, because rashomonic view-points might exist but in medicine we do have gold standards Randomized clinical trials and guidelines.
A health article which relies on opinion without reference to RCT and guidelines is not a good article in my opinion.
See for example the journalist vidya krishnan who writes at Hindu, she has been covering TB in for a decade now but in none of the articles(1,2,3,4) there is mention of WHO restriction or bad results of single phase Bedaquiline study while even benefits in observational MSF retrospective(not even prospective cohort ) are highlighted.
You can see the activism instead of objective journalism in her tweets on TB as well.
You could argue that she might have not known the facts, but
- It is her job to know facts
- She has been purposefully ignoring these facts as i have been pointing out to these issues for past 6 months to her on twitter in response to her activism
If it were not for her support of compulsory forced license for Bedaquiline, one would have thought Vidya was journalist directly acting under influence of manufacturer of Bedaquiline.
I reiterate , i fully support her advocacy of use of BDQ in XDR TB patients , moving away from donation based compassionate use model, but have serious concerns with her fear mongering in all cases of Drug resistant TB
Q. You are being very harsh on her, many other journalists do this.
A. It is true that even others do it, consider this case in which a girl of XDR TB correctly sued the government and got Bedaquiline on basis of her domicile status ( remeber WHO recommends BDQ for XDR TB not for routine MDR ones).
now Scroll rightly reported the story but again wrongly claimed that 1.3 lakh people are in need of medication and are being denied.
Q. How do you judge a news article for objectivity then?
I rely upon criteria established by a leading health journalism site which reviews health stories for their accuracy by gary schwitzer a highly respected health journalist in USA.

Now as an exercise to the reader, in light of guidelines and RCT i have presented i would let them read all articles i have referenced , I fear most of the articles of Hindu and scroll on TB would fail this test.
Consider this in this story the two piece of documents with highest grade of evidence are single center phase 2 RCT and WHO guideline , yet they have almost been never cited in these articles.
I think journalists should start reading evidence synthesis in medicine or start how to read RCT literature instead of reading dictations from motivated activists, five star doctors or pharma companies
Q, But the government buys expensive drugs for rare genetic diseases as well why not Bedaquiline?
A. It is true , i have flagged this issue as well. Even advanced countries like UK struggle with moral dilemma to allow these medicines or not. In my opinion an NHS like trust should be formed in India and only drug which meet on cost-effectiveness and safety criteria on a consistent and graded basis should be allowed. but as we all know health is a state subject in India and many government officials and pharma companies make a lot of money by getting their drug approved over others.
you frequently see a 10 doses of a drug worth 30000 each with good but non-dramatic efficacy approved in hospitals, but 30-40 paise proven tablets like lasix,aspirin missing because lack of economic incentive. However i am a doctor and not a public health specialist and not knowledgeable enough to comment on all this.
Q .I read a study which claims Bedaquiline is cost effective in MDR TB
A. The study
a) doesn’t include mortality imbalance seen in original phase 3 trial
b) is sponsored by Janssen itself
c) has UK based assumptions
I feel these three reasons are sufficient to indicate why it cant be applied in India in present state.
Q. You are a pessimist , when star docs like Dr Udwadia and Dr Furin are speaking for scaling up BDQ use , why are you protesting?
A Reputation is no substitute for evidence in field of Medicine.
If Dr. Furin and Dr Udwadia wish to speak for XDR patient ,I will go all distance with them..
Most doctors start out in activists mode only, as advocates for their patients regardless of guidelines.
I was one such person. When i started out using intensive insulin therapy in ICU and River’s trial for sepsis patients were like Religion for me. It was despite the fact that all of them were based on single center phase 2 study only
The same set of arguments: We should not wait for higher bar evidence as these patients are very sick, we will lose a lot of patients was touted.
I used to refer to my older faculty who resisted this as muggles.
Most Emergency medicine and critical care guidelines adopted it, when the results of large phase 3 multi country and they showed higher mortality in patients when we used these protocols in these cases. We ended up killing 15% more patients .
Now i am a bit older and i think my teaching faculty was right in this particular case and i am harangued by them whenever them meet me as “Insulin wala ladka”
Critical care also has learnt its lesson when today high dose IV vitamin C which is cheap, does not have any serious side effects is touted as next miracle , they have told Dr. Marik to go ahead and do a trial first .
Most innovators are passionate believers in use of drugs for their patients,consider the number of times game-changer has been used for drugs/medication in past 10 years yet we have not seen so many miracles.
you can see here and here the amount of medical reversal we saw in last decade.

Also see here a great article by Ioannidis, about reading medical literature with care as most published observational,single center phase 2 studies will show inflated effects which would not be replicated in later studies,for a conceptual view.
Q. what is your credibility in tarnishing these well respected newspapers?
A. One of the reasons i wrote this article was the fact that these papers have high credibility more so in field of health and science. My point is that when they speak from the pulpit which have been provided to them they should do it with responsibility. while write a blog which few read or rant on twitter, they influence thousands.
Think about it it shouldn’t take a lawyer like prashant reddy or a doctor like me to blow out holes in a seasoned journalists story and they shouldn’t have to do the work of journalists otherwise what is the point?
Apart from it I am a doctor, I worked as pulmonologist treating TB patients for 3 years , am a TB survivor myself and had professional and personal debacles because of TB(didnt want to create an emotional anchor by putting this information at top) and have presented my MD thesis in CBNAAT in diagnosis of drug resistance in Tuberculosis cases.
Current i work in cardiology, have not been treating TB for three years , but have been giving cardiac clearances for Bedaquiline in TB patient. In my experience 2 out of 50 patients in Bedaquiline had QT prolongation for which we stopped the drug while no cardiac event was reported to me. But you see it is anecdotal and I would like to form my judgment based on an RCT.
KEY TAKEAWAYS:
- It is role of journalists to evaluate evidence for a drug/intervention/policy their news article should not read as PR campaign of activists, pharmaceutical companies or five star doctors.
- Journalists and common people should be suspicious of opinion presented without evidence
- There should be inquiry into the fact that if compassionate use Program in fact an open label clinical trial
- Medical reversals in last decade tell us to be careful with assessment of new drugs and technologies and keep the bar high even when they are pushed under the garb of patient benefit.
If you have read so far, thank you for your patient reading, kindly address your doubts here in comments section or at my twitter feed : @anupampom
Pingback: Finally, The Hindu acknowledges the clinical trials issues regarding new TB drugs but through a rose-tinted glasses | SpicyIP